Interventional Analgesia Part II
As veterinary pain management becomes more like human pain management in offering patients a broader range of strategies for the treatment of acute and chronic pain, the interventional strategies and the techniques described below will become more commonplace.
For a detailed discussion of the pathophysiology of pain, please refer to part I of this series on interventional analgesia.
What type of imaging or techniques are involved in interventional pain diagnosis and management?
Radiography is useful as a baseline screening tool for skeletal interventions. However, newer modalities such as computed tomography (CT) allow even better resolution of boney structure and disruption with spatial resolution; CT is particularly useful in massive tissue damage such as that induced by trauma in small animals whereby multiple body cavities can be “scanned” without regard to radiographic technique.
Fluoroscopy has proved very fruitful for larger joint or facet injections, epidural and spinal injections, but requires ionizing radiation as well.
Magnetic resonance imaging (MRI) and functional MRI has been ultimately linked to incredible advances in pain management, specifically concerning human neurologic disease/neuropathic pain and soft tissue disease (such as fascial, tendon, ligament and intra-articular/periarticular structures) but both require general anesthesia and cost is considerable for veterinary patients.
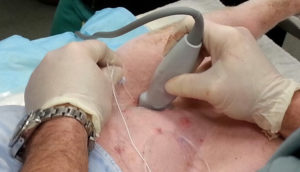
Fig. 1: Ultrasound guided femoral nerve block being performed via inguinal approach using ultrasound, in a patient undergoing cruciate reparative surgery.
In recent years ultrasonography has been recognized as a non-invasive, practical method for localizing “soft tissues”, and in
particular analgesia-wise, peripheral nerves, plexi, difficult joints (facets), tendons, and even epidural spaces; this modality vastly improves block success and safety. It is my choice of diagnostic modality for most analgesic interventions. Ultrasonography has many advantages in that it is less cumbersome and faster than fluoroscopy and CT for perineural or soft tissue visualization. This diagnostic modality can be utilized in awake or lightly sedated patients, is very mobile, and the cost of the procedure is significantly less than other imaging modalities such as MRI or fluoroscopy studies, making it widely accessible to assess both soft tissue and nerve structures, especially on recheck exams.
Neurolocation
Neurolocation (aka electrostim-location, electrolocation, neurostim-location) is a technique whereby electric current is directed onto nerve fibers suspected of supplying a painful, or potentially painful (upcoming surgery), area with stimulation. The nerve will depolarize and contractions of the innervated muscle bodies will occur. Neurostimulation techniques have been identified for major nerves, nerve roots, and plexi in both human and veterinary patients. These techniques have guided perioperative pain control with local anesthetics for the past decade in veterinary medicine. Recent developments in nerve stimulation location include the use of a peripheral nerve stimulator to determine the location of a peripheral nerve with purely sensory fiber components, percutaneous guidance to guide actual initial needle insertion, and even stimulating catheters for continuous infusion of substances.
Neurostimulation location requires an inexpensive nerve stimulator, specialized sheathed needles, and catheters designed to deliver a low enough current to assure proximity to nerves, nerve roots, plexi, or ganglia.
To treat the intended issue, and avoid toxicity, extraneural structural damage and intraneural injections, the goal of any local or regional anesthetic technique should be to use the lowest appropriate volume of local anesthetic agent as close as possible to the nerve of question (but not within this nerve). One way to avoid intraneural injection is to locate with electrostimulation.
Peripheral nerve stimulators used for nerve location should have short pulse durations such that motor responses can be elicited without causing discomfort or pain (in human pain management, these are used in awake patients without causing discomfort). The lower the intensity needed to stimulate the nerve, the closer the needle tip will need to be, and hence the more accurate and effective the blockade. Insulated needles should be used when performing nerve stimulation-guided blocks. These needles typically have a Teflon coating along the shaft of the needle with an exposed tip. Neurostimulation location occurs only when current intensity is applied by the needle electrode and the tip is sufficiently close the nerve; consequently, a muscle contraction becomes evident.
Ultrasound Guided Location
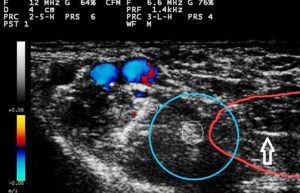
Fig. 2: Ultrasound guided femoral nerve block. Arrow shows sheathed needle penetrating vastus medialis muscle (outlined by red line). Small white circles show femoral nerve (larger white circle) and saphenous nerve (smaller white circle). Blue circle outlines deposition of injectate.
Utilizing ultrasound, one must interrogate the proposed area using both grayscale and Doppler imaging; vessels should be noted and avoided. A general site of approach is then approximated and bony landmarks/skin may be marked if desired. Needle and syringe selection is joint-, tendon- and nerve-specific and should be prepared prior to injection. The beam should be directed obliquely at the needle and advancement is directly visualized. The tip of the needle is directed underneath the ultrasound transducer. The needle may be started cranial or caudal to the transducer. The needle is advanced through skin and subcutaneous structures into the given area, and sampling may occur prior to injection. During the injection, circumferential spread of drug around a nerve or tendon, ligament, or other pathologic structure confirms appropriate placement. A special ultrasound visible needle (echogenic) may be used to improve visualization, although scoring the shaft of the distal needle with a scalpel blade has a similar effect.
Most practitioners trained in ultrasound can visualize soft tissue structures well due to obvious structure, shape, and echogenicity of various problem areas. In longitudinal view, muscles appear as relatively hypoechoic structures with fine, oblique echogenic striations representing fascia. Likewise, tendons are hyperechoic and composed of multiple organized parallel lines surrounded by a minimal amount of anechoic fluid. The tendon sheath appears thin and hyperechoic at the peritendon-superficial soft tissue interface. It is imperative that the transducer remain parallel to the linear fiber pattern of the tendon to prevent artifact.
Ultrasound identification of nerves in cross section can be challenging. They often appear round to ovoid and demonstrate different echogenicity. They are hypoechoic or dark, showing the neural component, or hyperechoic/bright showing the connective tissue component. Needle imaging during advancement provides real-time visual guidance to minimize random needle movement as the block needle is advanced toward the target nerve.
A recent conference dedicated to ultrasound and e-stim location, and application of advanced locoregional analgesic techniques in veterinary patients was hosted by the University of Florida’s College of Veterinary Medicine in Gainesville. The conference was attended by human and veterinary technicians, anesthesiologists and practitioners. There is an expectation that this conference will be held regularly going forward.
Examples of Image Guided Analgesic Interventions
A plethora of injectable drugs are used in interventional pain management techniques. Choice of agents and dilution are often unique to each blockade, and dependent on the target tissue (nerve, plexus, fascia, tendon, or ligament); these are discussed at length below. There can be neuraxial (epidural or spinal), perineural (near to major nerves and plexi), intraarticular, tendon or trigger application, and there may be a single shot (one time) or continuous application (usually through a catheter). Substances can be used for immediate (usually acute, intensive care, or surgical), long term (repository or encapsulated), or even (semi-) permanent neurolytic effect.
Intraarticular Interventions: Analgesic intra articular interventions in veterinary medicine are required for multiple reasons including:
• Ligamentous injury
• Immune-mediated or degenerative arthropathy (eg. osteoarthritis in dogs, facet disease in horses)
• Perioperative analgesia
• Developmental disease (eg. osteochondritis dissecans in horses and dogs, elbow and hip dysplasia in dogs)
Veterinarians have used “blind” injections as veterinary education has historically lacked image guidance for joint therapies and relied on boney landmarks. Ultrasonography seems to be clearly improving a majority of equine facet intra- and periarticular interventions.
• Local anesthetic use in intra-articular administration has recently been questioned due to chondrotoxicity potential. Antibiotics, particularly aminoglycosides (gentamicin, amikacin), whether in parenteral or encapsulated form can be added to intra-articular or tendon injections to avoid infection, but are rarely required.
• A review of pharmacologic agents for both diagnostic and therapeutic use is available, as well as reports of use for surgical pain, septic arthritis, post-surgical healing, chronic degenerative disease; most recent reports involve cell based regenerative therapies, hyaluronate, triamcinolone, local anesthetics and opioids.
• Viscosupplementation refers to the use of substances such as hyaluronate delivered intra-articularly; this intervention for equine performance preceded use in humans and dogs. Although the actual indwelling time of injected hyaluronate is days, the beneficial effects are variable, lasting from days, to months. Some argue that the use of autologous biologic therapies such as cell based (embryonic and mesenchymal stem cells, fibroblast and tenocytes), or factor based (platelet-rich plasma (PRP) and IL-1 receptor antagonist protein (IRAP), has driven clinical usage to the point that it has outpaced, or perhaps even bypassed, scientific investigation into their use.
Peri-tendinous Interventions: Tendon injuries occur commonly in both horses and dogs and analgesic interventions are required commonly for conditions such as trauma and injury, overuse and strain, and occasionally systemically displaced infection.
• In dogs, shoulder (biceps, supra- or infraspinatus) tendonitis, calcaneal tendonitis and tendon rupture, patellar tendonitis secondary to cruciate damage and repair, or patellar luxation, sesamoiditis, and carpal/tarsal injuries in the working dog frequently require interventions. Iliopsoas muscle and musculotendinous injury should also be included in this group.
Treatment for canine tendon disease may involve ultrasound guidance, and in contrast to equine desmopathies (which currently rely heavily on regenerative therapies), often involves steroids, homeopathic preparations (Arnica, Traumeel), sterile water, dextrose, glycerin, phenol, or even local anesthetics, in addition to platelet rich plasma (PRP) and other cell based therapies.
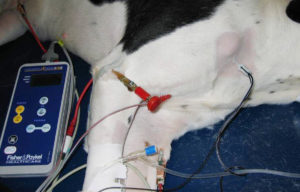
Fig. 3: Neurostimulation location of brachial plexus for delivery of neurolytic block in a canine patient. Blue arrow: Nerve location needle in brachial plexus of right forelimb.
Regenerative and cell based therapies seem most utilized when considering intra-articular or tendon based interventions in veterinary medicine. Scientific evidence indicates that PRP can provide a scaffold and growth factor concentrate to enhance the cellular repair of musculoskeletal lesions in both soft and orthopedic tissues. PRP is an attractive product because of its autologous nature, non-invasive collection process and rapid preparation.
Therapeutic Perineural Interventions: Locoregional or nerve blockades are used in both small and large animal arenas for treatment of acute and surgical pain. Using various combinations of agents (mostly local anesthetics, with or without saline, ketamine, or opioids), they reduce the need for inhalant, improve pain relief, permit surgery without need for heavier agents, and promote quicker recovery. Blind or non-guided peripheral nerve blocks have been used diagnostically in the equine patient, and recently in the canine patient as well.
Therapeutic nerve blocks have been used in chronic pain management in humans through several venues, including medial branch blocks for chronic lower back pain, neurolytic blocks for cancer pain, and specific or selective nerve, nerve root, or plexus blocks for chronic post-traumatic or neuropathic pain.
Chronic pain or palliative nerve blockade deserves attention in veterinary medicine. Reports of neurolysis in veterinary patients are few, but reports using resinferintoxin perineurally showed promising results in reducing inflammatory hyperalgesia. Risks of (semi-) permanent neurolysis include impermanence, neuritis, neuroma formation, motor deficits, and structural/functional damage to non-neural structures. Due to plasticity and the fact that cell bodies are usually spared, pain relief is solid but rarely permanent, averaging somewhere between 2-30 weeks in patients with stable disease.
Neurolytic blockade using alcohol, ammonium salts, phenol, glycerol, and hypertonic saline have all been utilized to produce Wallerian degeneration when administered neuraxially or perineurally in patients with limited life expectancy, usually those suffering from intractable pain and cancer pain.
Written by Andrea Looney, DVM, DACVAA, CCRP, DACVSMR